In the early 1970s the carcinogenicity of vinyl chloride usually called vinyl chloride monomer or vcm was linked to cancers in workers in the polyvinyl chloride industry.
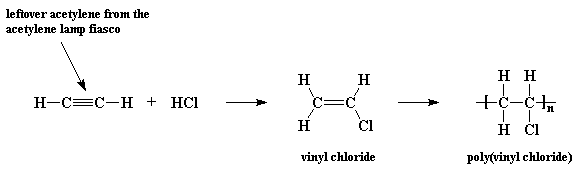
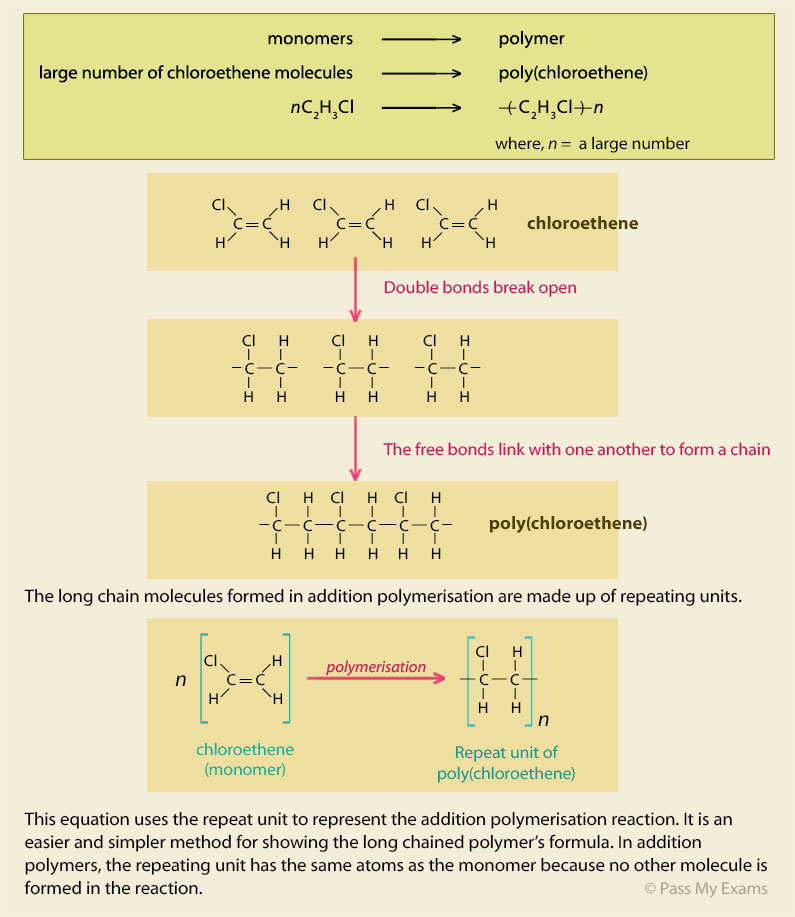
Polymerisation of vinyl chloride equation.
Pvc is the widely third most large using synthetic polymer in world followed by polyethylene polypropene.
Pvc is used in the manufacture of numerous products including packaging films and water pipes.
Chain reaction polymerization sometimes called addition polymerization requires an initiator to start the growth of the reaction.
Polyvinyl chloride pvc chemical formula.
Polyvinyl chloride is produced in an addition polymerisation reaction using the chloroethene vinyl chloride monomer.
The largest family of polymers 3 vinyl polymers are produced by chain polymerization reactions a good example is the free radical polymerization of styrene which is initiated by a free radical r that reacts with styrene.
Poly vinyl chloride is also known as poly vinyl or vinyl as abbreviated known as pvc.
Vc vc αd 1 vc β βd 2 and vc d 3 were used to study the reactivities of the hydrogen atoms in the polymerization and the β hydrogen atoms contributed to the chain transfer.
Pvc vinyl chloride is an organohalogen compound that has important industrial applications.
Specifically workers in polymerization section of a b f.
Goodrich plant near louisville kentucky were diagnosed with liver angiosarcoma also known as hemangiosarcoma a.
Mol view music credit.
It can be produced by three different processes.
When treated with certain catalysts vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride or pvc.
Polymerization of vinyl chloride vc was studied.
Polyvinyl chloride is a white rigid quite brittle solid.
Chemical and physical methods were used to observe irregular structures such as branching.
Polyvinyl chloride is a white brittle solid.
Poly vinyl chloride pvc is one of the most broadly produced thermoplastic materials in use today which may be employed to obtain among other things latex coatings adhesives pipes and paints nass 1976.
Emulsion suspension and bulk polymerization.
This polymerisation reaction proceeds by a free radical mechanism.
Being a very well known memb r of the family of vinyl polymers.
Pvc ranks as the second most important polymer after ethylene.
Natural evolution of hcl from vc occurred in the polymerization.
The term polyvinyl chloride or pvc indicates homopo1ymers of vinyl chloride and incorrectly copolymers containing amow1ts of vinyl idene chloride vinyl acetage ethylene propylene or acrylates.