It is resistant to acids and alkalies and has good electrical insulation.
Polystyrene what group is on the vinyl monomer.
Pitsikalis in reference module in chemistry molecular sciences and chemical engineering 2013.
Polystyrene is a vinyl polymer.
It therefore finds application in packaging.
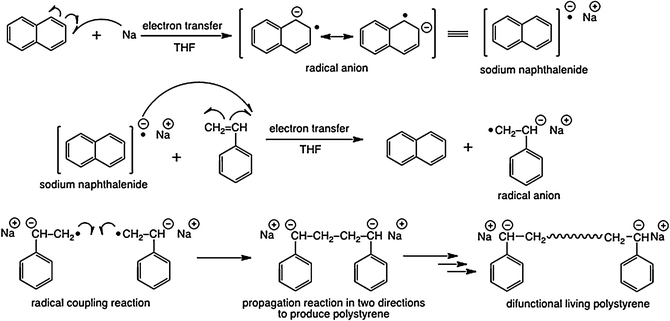
Polystyrene is a polymer obtained by the polymerization of styrene monomer.
Structurally it is a long hydrocarbon chain with a phenyl group attached to every other carbon atom.
Polystyrene is a vinyl polymer.
Polystyrene is made from styrene r c 6 h 5.
Polypropylene from propylene r ch 3.
Polystyrene is an addition polymer that results when styrene monomers interconnect polymerization.
Polystyrene is produced by free radical vinyl polymerization from the monomer styrene.
Since only one kind of monomer is used in its preparation.
In the polymerization the carbon carbon π bond of the vinyl group is broken and a new carbon carbon σ bond is formed attaching to the carbon of another styrene monomer to the chain.
Structurally it is a long hydrocarbon chain with a phenyl group attached to every other carbon atom.
78 79 these monomers can be classified in two main categories.
Vinyl polymers are the most common type of plastic.
Polyethylene r h.
1 vinyl monomers for which the reactive end group is a carbocation and 2 heterocyclic monomers bearing one or two heteroatoms.
Important examples can be distinguished by the r group in the monomer h 2 c chr.
Monomers bearing electron releasing groups are susceptible to cationic polymerization.
Polystyrene is also used in toys and the housings of things like hairdryers computers and kitchen appliances.